Lastly, researchers might have methods to fight a lethal respiratory sickness.
The illness just isn’t the flu or COVID-19, however respiratory syncytial virus, or RSV.
The virus was first recognized in 1957 in Baltimore, however it has in all probability been round for millennia, says Jim Boonyaratanakornkit, a virologist and transplant immunologist on the Fred Hutchinson Most cancers Heart in Seattle. “It’s all the time been a plague for the very younger and the very previous,” he says.
In Europe, individuals age 60 and older might quickly get some safety towards the virus. An advisory committee really helpful April 26 {that a} vaccine towards RSV made by the pharmaceutical big GSK ought to be permitted to be used. That call will go to the European Fee for last approval.
No RSV vaccine has been permitted in america. And there aren’t any particular antiviral medicines to fight RSV, and just one preventive remedy — a monoclonal antibody — is reserved for a small variety of infants at excessive danger of extreme illness. However that would quickly change. A number of corporations now have medical trial knowledge suggesting that their varied vaccine candidates or lab-made monoclonal antibodies can defend towards RSV’s worst penalties.
These remedies are geared, partly, towards the youngest kids. Folks of all ages can contract the virus, however it tends to hit the youngest the toughest. In 2019, RSV triggered an estimated 33 million infections worldwide in kids ages 5 and youthful. About 3.6 million kids have been hospitalized and greater than 100,000 died, researchers reported final 12 months within the Lancet. Their calculations revealed that RSV causes 1 in each 28 deaths of youngsters 28-days- to 6-months-old.
“Because of this we should always care,” Penny Heaton of Janssen Prescription drugs, an organization owned by Johnson & Johnson, mentioned April 4 on the World Vaccine Congress, held in Washington D.C. Vaccines and antibodies would possibly have the ability to forestall a few of these deaths. “That is the superb influence that … RSV vaccines [and] monoclonal antibodies … can have on the well being of youngsters globally.”
Nevertheless it’s not simply the 5-and-under crowd who will profit. Their grandparents, great-grandparents and different older adults might quickly get RSV pictures. That might imply an easing of the burden of RSV within the two teams hit hardest by the virus.
Two corporations — GSK, previously GlaxoSmithKline, and Pfizer — are near getting approval from the U.S. Meals and Drug Administration for his or her RSV vaccines. Moderna isn’t far behind with an mRNA-based RSV vaccine. In the meantime, Danish firm Bavarian Nordic expects outcomes from a medical trial quickly for its vaccine, and several other different corporations even have RSV vaccines within the works.
Different corporations are growing monoclonal antibodies to present to infants and infants as preventives towards RSV. These lab-made antibodies aren’t vaccines, however a dose given earlier than the beginning of RSV season might briefly defend susceptible infants from changing into very sick in the event that they get contaminated.
There are such a lot of choices on the horizon that Janssen determined to bow out. “As we regarded throughout our portfolio, and we regarded throughout the RSV panorama,” Heaton mentioned, “we have now made the choice to discontinue our RSV program,” and to cease a late-stage medical trial. The corporate additionally introduced the choice in March, saying it needed to deal with unmet medical wants. RSV, it appears, might quickly now not match that invoice.
For now, the virus remains to be very a lot an issue. Often, RSV season begins in October and peaks in December or January — no less than in america. It usually ends in April.
The virus nearly vanished within the winter of 2020–21. In that first 12 months of the COVID-19 pandemic, RSV-positive PCR assessments by no means rose above 3 p.c — the U.S. Facilities for Illness Management and Prevention’s threshold for an epidemic. Earlier than the COVID-19 pandemic, RSV positivity charges peaked every season at about 13 to 16 p.c. RSV’s disappearance was thanks principally to precautions, together with social distancing and mask-wearing, put in place towards COVID-19.
However the virus rebounded, showing in the summertime of 2021, researchers report April 7 in Morbidity and Mortality Weekly Report. That 12 months RSV season shifted, beginning in Might. It hit its zenith in July and resulted in January 2022.
The 2022–23 RSV season shifted nearer to the prepandemic sample however was nonetheless early. The 2022 season began in June and peaked in November with a positivity charge of 19 p.c. The season ended someday between December and February, relying on which a part of the nation you reside in. This newest RSV season hit youngsters arduous, overwhelming kids’s hospitals in some locations.
Nobody is aware of when the subsequent RSV season will start. However a few of the vaccines and coverings in improvement might be prepared for deployment this fall, doubtlessly lowering the variety of physician visits, hospitalizations and deaths from the infections.
Right here’s a breakdown of the vaccine and remedy candidates.
What’s within the vaccines?
Three corporations — Pfizer, GSK and Moderna — have primarily based their vaccines on certainly one of RSV’s proteins. That protein, referred to as the F protein, sits on the virus’s outer membrane and helps it fuse to human cells. The F protein is a shapeshifter. Earlier than fusion, it seems to be like a rounded knob. After fusion, it resembles a needle or pointy tower.

A decade in the past, researchers on the U.S. Nationwide Institutes of Well being discovered that locking the protein into its prefusion knob state causes the immune system to react extra strongly than to the virus’ shapeshifting type. All three corporations use some model of the F protein locked within the prefusion state of their vaccines. Pfizer described the nitty-gritty particulars of constructing this stabilized F protein April 6 in Science Translational Drugs.
Pfizer and GSK’s vaccines include the protein itself. Moderna’s candidate, like its COVID-19 vaccine, is an mRNA vaccine that tells the physique to provide the protein.
Bavarian Nordic is taking a distinct method. The corporate makes a vaccine that works towards each smallpox and mpox, previously referred to as monkeypox (SN: 12/12/22). That vaccine is reside vaccinia virus, a pox virus that has been engineered in order that it may possibly’t replicate properly within the physique. Many vaccines, together with these towards flu, measles and hen pox are additionally such reside attenuated, or weakened, viruses.
For its RSV vaccine, Bavarian Nordic engineered the vaccinia virus to make 5 of RSV’s proteins, together with the F protein. On the World Vaccine Congress, one particular person requested whether or not such a hybrid virus would possibly give safety towards RSV, mpox and smallpox. However the firm has no knowledge but to counsel the vaccine would defend towards all three, Peter Costa, Bavarian Nordic’s U.S. medical affairs regional lead, mentioned April 5 throughout a session wherein a number of corporations introduced knowledge on RSV vaccines and monoclonal antibodies.
One other firm, Codagenix, is working with the RSV virus itself, tinkering with its genetic instruction ebook to make the virus unable to trigger illness. For its vaccine, the corporate’s researchers launched greater than 1,000 mutations in a single gene to gradual replication of the virus, says Jeffrey Fu, the corporate’s chief enterprise officer. These mutations change the virus’s RNA however don’t alter the amino acids in its proteins.
“We’re in a position to design viruses … that look equivalent or practically equivalent to the true virus,” Fu instructed me. As a result of the virus replicates slowly, it doesn’t trigger illness however stimulates an immune response. The vaccine can be given as nostril drops, in contrast to the opposite vaccines, that are given as pictures.
Codagenix plans to begin testing its vaccine’s security at a low dose in wholesome 5-year-olds this spring. If protected, the corporate needs to step by step enhance the dose in addition to start testing the vaccine candidate in youthful kids, working all the way down to 6-month-olds.
Pfizer’s vaccine is being examined for its potential to guard newborns, infants and older adults. The remainder of the businesses’ vaccines are to be used in older adults.
How properly do the RSV vaccines work in newborns and infants?
Solely Pfizer is testing a vaccine to guard newborns. And it doesn’t give pictures to infants. As an alternative, the corporate gave its vaccine candidate to greater than 7,300 wholesome pregnant girls age 49 or youthful. The concept is that the mom will produce antibodies towards RSV that may switch by the placenta to the child. These antibodies would give infants momentary safety towards the virus within the particularly susceptible first six months of life.
Most youngsters get contaminated with RSV by the point they’re 2 years previous. The illness could also be only a coldlike sickness for a lot of youngsters, with a runny nostril, lower in urge for food and a cough. However wheezing or problem respiratory can even happen, and really younger infants would possibly pause respiratory for greater than 10 seconds. RSV can even result in extra extreme sicknesses together with bronchitis and pneumonia.
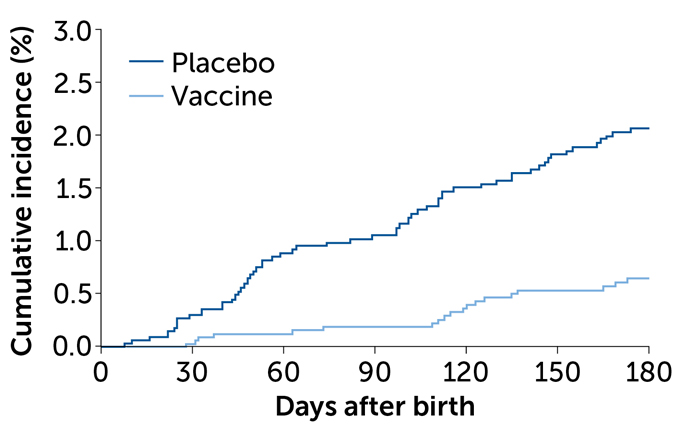
Pfizer’s technique to guard infants from these penalties of RSV appears to have labored. Within the 90 days after start, six infants born to mothers who received the vaccine had extreme RSV lung infections requiring medical consideration. Within the placebo group, 33 infants had extreme lung infections. That’s a vaccine efficacy of 81.8 p.c, researchers report April 5 within the New England Journal of Drugs.
Over time, that vaccine efficacy slipped a bit as moms’ antibodies wore out. Inside six months after start, 19 infants of moms within the vaccine group and 62 infants of moms within the placebo group developed extreme lung infections, a vaccine efficacy of about 69 p.c.
The vaccine didn’t meet statistical standards for stopping less-severe lung infections, however there have been about half the variety of such infections in infants whose moms received the vaccine in contrast with infants whose moms received a placebo. Inside 90 days after start, 24 infants within the vaccinated-mom group and 56 within the placebo group received lung infections. That’s roughly 57 p.c efficacy. That efficacy towards less-severe lung infections additionally slipped barely, to 51 p.c, inside 180 days of start.
The vaccine stored infants out of the hospital too. Within the first 90 days of life, the vaccine’s efficacy was nearly 68 p.c at stopping hospitalization, falling to about 57 p.c efficacy inside 180 days of start.
Just like the COVID vaccines and most vaccines for respiratory sicknesses, the vaccine wasn’t nice at stopping an infection (SN: 5/29/22). The vaccine’s efficacy towards any RSV sickness requiring medical consideration was about 39 p.c within the first three months of life and 38 p.c within the six months after start.
As for security, there weren’t uncomfortable side effects past what researchers anticipated; some ache on the injection website, some muscle aches. “This was a maternal trial, so clearly mothers have been drained [and] had complications. You’ll be able to see that from the placebo numbers,” Barbara Pahud, the medical lead for Pfizer’s ongoing examine, mentioned on the vaccine congress. No severe security considerations brought on by the vaccine appeared through the trial in both moms or infants.
Pfizer is constant to comply with the kids and could have two years’ value of knowledge to current forward of an FDA assembly to debate the vaccine’s approval in August, she mentioned.
Codagenix has knowledge from lab animal research suggesting its vaccine can set off the manufacturing of protecting antibodies. However its trial in kids is simply getting began, and there aren’t any outcomes to report but.
Do monoclonal antibodies assist defend newborns and infants?
Giving monoclonal antibodies to newborns and infants would possibly assist towards RSV too. Like antibodies handed from moms to infants, lab-made antibodies focused towards the virus’s F protein are exhibiting some indicators of success at defending infants towards RSV.
One monoclonal antibody, palivizumab, was permitted by the FDA in 1998. However that antibody is used just for infants on the highest danger of extreme sickness from RSV. That features infants born prematurely, these with a persistent lung situation referred to as bronchopulmonary dysplasia and infants with sure coronary heart circumstances. That’s solely a fraction of the infants born in america annually. And palivizumab isn’t out there in many of the low- and middle-income nations the place the virus is the largest drawback, Codagenix’s Fu says.
What’s extra, palivizumab doesn’t final lengthy. Susceptible infants want an injection each month throughout RSV season. And at greater than $1,800 per dose, the medicine is dear.
Some corporations have been engaged on stronger, longer-lasting monoclonal antibodies to be used in infants and infants. Sanofi and AstraZeneca teamed as much as make one referred to as nirsevimab. That antibody was permitted to be used within the European Union and the UK in November. It’s being thought of for approval in america.
In a medical trial, about 2,000 newborns received an injection of nirsevimab and about 1,000 infants received a placebo. By way of 150 days after getting the shot, the antibody’s efficacy towards hospitalization was 76.8 p.c, researchers report April 5 within the New England Journal of Drugs.
Merck additionally has a long-lasting monoclonal antibody being examined in medical trials. Outcomes from these research aren’t but out there.
Do the vaccines defend older individuals?
Infants, toddlers and preschoolers are maybe the inhabitants that individuals fear most about in relation to RSV, however the virus hits older individuals arduous too.
In america alone, between 60,000 and 160,000 older adults are hospitalized with RSV annually, and 6,000 to 10,000 die, the CDC estimates.
In 2019, an estimated 5.2 million individuals ages 60 or older caught RSV in high-income nations, researchers reported final 12 months in Influenza and Different Respiratory Viruses. Of the 5.2 million instances, 470,000 individuals landed within the hospital, with about 33,000 of these dying, the researchers estimate. The examine regarded solely at high-income nations, together with america, Canada, South Korea, Japan and a few European nations. But when the child knowledge are any indication, the dying charge for older people might have been a lot larger had individuals in low- and middle-income nations been included.
“It is a comparatively unrecognized an infection in grownup populations,” Edward Walsh, an infectious ailments physician on the College of Rochester Medical Heart in New York, mentioned April 5 on the World Vaccine Congress.
Walsh was one of many lead researchers testing Pfizer’s vaccine for older adults. He and colleagues carried out a big trial involving greater than 34,000 individuals ages 60 and older in Argentina, Canada, Finland, Japan, the Netherlands, South Africa and america. Half received Pfizer’s vaccine candidate, and half received a placebo.
The trial began in August 2021. Although RSV reemerged to contaminate quite a lot of kids that 12 months, it didn’t come again in prepandemic numbers in older adults, particularly not within the over-60 crowd, Walsh mentioned. “They continued to steer clear of grandchildren, steer clear of crowds and put on masks,” stopping transmission.
Walsh instructed me later that he and colleagues discovered solely 16 p.c of prepandemic numbers of infections in older adults within the Rochester space through the examine interval. With such low numbers of infections, “we have been very nervous that [the trial] wasn’t going to point out us something.”
The Rochester space was simply certainly one of 240 websites within the examine. With the knowledge collected from every website, the researchers had sufficient knowledge to calculate the vaccine’s efficacy. The researchers thought of completely different ranges of severity primarily based on whether or not the virus contaminated the lungs and what number of signs, similar to cough, wheezing or shortness of breath, that members reported.
Within the placebo group, 14 members developed lung infections with three or extra signs. Solely two members who received the vaccine developed that stage of sickness for an efficacy of 85.7 p.c, Walsh and colleagues report April 5 within the New England Journal of Drugs. Towards RSV lung infections with two or extra signs, the vaccine had an efficacy of 66.7 p.c, with 33 instances within the placebo group and 11 within the vaccine group.
These efficacy numbers are decrease however just like these reported by GSK on February 16 within the New England Journal of Drugs. However outcomes from the vaccine trials will not be instantly comparable as a result of they have been carried out in several nations and used completely different measurements.
GSK examined its vaccine on about 25,000 members age 60 and over from 17 nations throughout 5 continents. Within the trial, seven individuals who received the corporate’s vaccine and 40 who received a placebo developed RSV lung infections with two or extra signs lasting no less than 24 hours. That’s an efficacy of 82.6 p.c. The European Medicines Company’s committee for human medicines really helpful the vaccine for approval on the energy of these knowledge.
Moderna hasn’t but revealed outcomes from its vaccine trial in a scientific or medical journal, however it issued a press launch in January with the numbers. The corporate gave its vaccine or a placebo to about 37,000 adults ages 60 and older in 22 nations. Solely 9 individuals within the vaccine group received lung infections with two or extra signs in contrast with 55 within the placebo group, for a vaccine efficacy of 83.7 p.c.
Bavarian Nordic gave wholesome 18- to 50-year-olds a shot of vaccine or placebo after which purposely gave them RSV in a human problem trial (SN: 2/18/21). The vaccine prevented symptomatic infections with 79 p.c efficacy, the corporate reported in a press launch in 2021. However that was amongst wholesome youthful adults who’ve much less danger of extreme problems than aged individuals do.
Bavarian Nordic additionally carried out a medical trial in america and Germany of 20,000 individuals age 60 and older. Outcomes of the examine ought to be out there later this 12 months, Costa mentioned.
With out masking and social distancing, RSV in all probability isn’t going to vanish once more. As for the brand new vaccines and monoclonal antibodies, they in all probability gained’t cease RSV’s unfold. However they might no less than defend essentially the most susceptible amongst us towards the virus’s worst results.